The modern periodic table, or the long form of the periodic table, are positioned in ascending order of the atomic numbers. This table is more accurate, and the prediction of the elements and their compounds can be made more exact. Also, this table can fix the limitations of Mendeleev’s periodic table.
Structure of the Modern Periodic Table
The long form of periodic table consists of,
- 18 vertical columns known as groups
- 7 horizontal rows known as periods.
Elements having the same electronic configuration are placed in the same group. Hydrogen could not be placed in the appropriate position in Mendeleev’s Periodic Table, which is fixed on the long form of the periodic table. Also, there were new elements yet to be discovered. Let’s look at the structure of the Long form of periodic table.
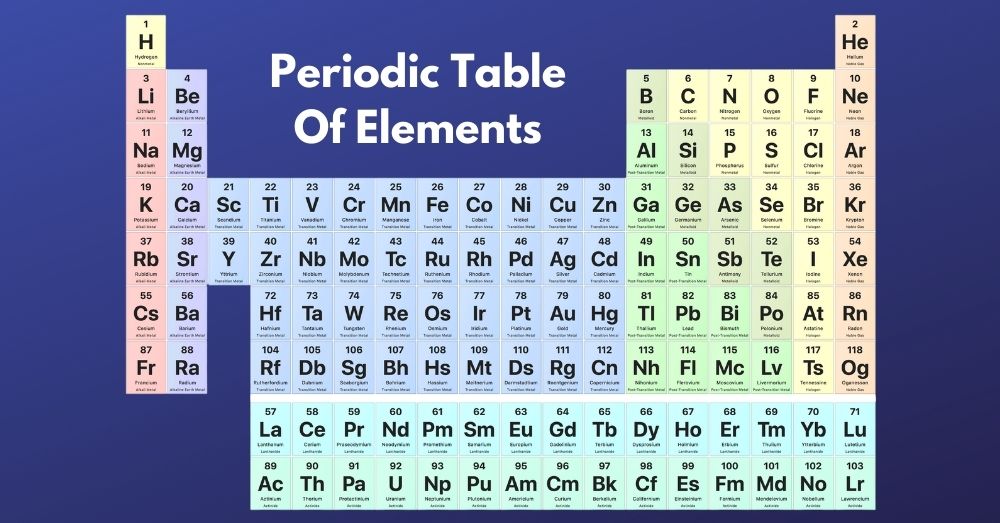
Neil Bohr is the scientist who proposed the long form of the Periodic Table. The long form of the periodic table is based on the atom’s atomic number, and hydrogen is the first element in the table.
The first period is the shortest in the periodic table with H and He elements, whereas the sixth period is from Cs to Rn and is the longest period.
Values of Elements in the Long form of Periodic Table
The long form of the periodic table based on the atomic number is given below. The table consists of elements and their Name, Atomic Number, Symbol, Atomic Mass, Electron Configuration, Electronegativity, Atomic Radius (Van der Waals radius), Ionization Energy, Electron Affinity, Oxidation States, Standard State, Melting Point, Boiling Point, Density Group Block, Year Discovered.
| Atomic Number | Symbol | Name | Atomic Mass | Electron Configuration | Electronegativity | Atomic Radius (Van der Waals radius) | Electron Affinity | Oxidation States | Ionization Energy | Standard State | Melting Point | Boiling Point | Density | Group Block | Year Discovered |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | Hydrogen | 1.008 u | 1s1 | 2.2 | 120 | 0.754 eV | +1, -1 | 13.598 eV | Gas | 13.81K | 20.28K | 0.00008988 g/L | Nonmetal | 1766 |
| 2 | He | Helium | 4.0026 u | 1s2 | 140 | 0 | 24.587 eV | Gas | 0.95K | 4.22K | 0.0001785 g/L | Noble gas | 1868 | ||
| 3 | Li | Lithium | 7 u | [He]2s1 | 0.98 | 182 | 0.618 eV | 1 | 5.392 eV | Solid | 453.65K | 1615K | 0.534 g/L | Alkali metal | 1817 |
| 4 | Be | Beryllium | 9.012183 u | [He]2s2 | 1.57 | 153 | 2 | 9.323 eV | Solid | 1560K | 2744K | 1.85 g/L | Alkaline earth metal | 1798 | |
| 5 | B | Boron | 10.81 u | [He]2s2 2p1 | 2.04 | 192 | 0.277 eV | 3 | 8.298 eV | Solid | 2348K | 4273K | 2.37 g/L | Metalloid | 1808 |
| 6 | C | Carbon | 12.011 u | [He]2s2 2p2 | 2.55 | 170 | 1.263 eV | +4, +2, -4 | 11.26 eV | Solid | 3823K | 4098K | 2.267 g/L | Nonmetal | Ancient |
| 7 | N | Nitrogen | 14.007 u | [He] 2s2 2p3 | 3.04 | 155 | +5, +4, +3, +2, +1, -1, -2, -3 | 14.534 eV | Gas | 63.15K | 77.36K | 0.0012506 g/L | Nonmetal | 1772 | |
| 8 | O | Oxygen | 15.999 u | [He]2s2 2p4 | 3.44 | 152 | 1.461 eV | -2 | 13.618 eV | Gas | 54.36K | 90.2K | 0.001429 g/L | Nonmetal | 1774 |
| 9 | F | Fluorine | 18.99840316 u | [He]2s2 2p5 | 3.98 | 135 | 3.339 eV | -1 | 17.423 eV | Gas | 53.53K | 85.03K | 0.001696 g/L | Halogen | 1670 |
| 10 | Ne | Neon | 20.18 u | [He]2s2 2p6 | 154 | 0 | 21.565 eV | Gas | 24.56K | 27.07K | 0.0008999 g/L | Noble gas | 1898 | ||
| 11 | Na | Sodium | 22.9897693 u | [Ne]3s1 | 0.93 | 227 | 0.548 eV | 1 | 5.139 eV | Solid | 370.95K | 1156K | 0.97 g/L | Alkali metal | 1807 |
| 12 | Mg | Magnesium | 24.305 u | [Ne]3s2 | 1.31 | 173 | 2 | 7.646 eV | Solid | 923K | 1363K | 1.74 g/L | Alkaline earth metal | 1808 | |
| 13 | Al | Aluminum | 26.981538 u | [Ne]3s2 3p1 | 1.61 | 184 | 0.441 eV | 3 | 5.986 eV | Solid | 933.437K | 2792K | 2.7 g/L | Post-transition metal | Ancient |
| 14 | Si | Silicon | 28.085 u | [Ne]3s2 3p2 | 1.9 | 210 | 1.385 eV | +4, +2, -4 | 8.152 eV | Solid | 1687K | 3538K | 2.3296 g/L | Metalloid | 1854 |
| 15 | P | Phosphorus | 30.973762 u | [Ne]3s2 3p3 | 2.19 | 180 | 0.746 eV | +5, +3, -3 | 10.487 eV | Solid | 317.3K | 553.65K | 1.82 g/L | Nonmetal | 1669 |
| 16 | S | Sulfur | 32.07 u | [Ne]3s2 3p4 | 2.58 | 180 | 2.077 eV | +6, +4, -2 | 10.36 eV | Solid | 388.36K | 717.75K | 2.067 g/L | Nonmetal | Ancient |
| 17 | Cl | Chlorine | 35.45 u | [Ne]3s2 3p5 | 3.16 | 175 | 3.617 eV | +7, +5, +1, -1 | 12.968 eV | Gas | 171.65K | 239.11K | 0.003214 g/L | Halogen | 1774 |
| 18 | Ar | Argon | 39.9 u | [Ne]3s2 3p6 | 188 | 0 | 15.76 eV | Gas | 83.8K | 87.3K | 0.0017837 g/L | Noble gas | 1894 | ||
| 19 | K | Potassium | 39.0983 u | [Ar]4s1 | 0.82 | 275 | 0.501 eV | 1 | 4.341 eV | Solid | 336.53K | 1032K | 0.89 g/L | Alkali metal | 1807 |
| 20 | Ca | Calcium | 40.08 u | [Ar]4s2 | 1 | 231 | 2 | 6.113 eV | Solid | 1115K | 1757K | 1.54 g/L | Alkaline earth metal | Ancient | |
| 21 | Sc | Scandium | 44.95591 u | [Ar]4s2 3d1 | 1.36 | 211 | 0.188 eV | 3 | 6.561 eV | Solid | 1814K | 3109K | 2.99 g/L | Transition metal | 1879 |
| 22 | Ti | Titanium | 47.867 u | [Ar]4s2 3d2 | 1.54 | 187 | 0.079 eV | +4, +3, +2 | 6.828 eV | Solid | 1941K | 3560K | 4.5 g/L | Transition metal | 1791 |
| 23 | V | Vanadium | 50.9415 u | [Ar]4s2 3d3 | 1.63 | 179 | 0.525 eV | +5, +4, +3, +2 | 6.746 eV | Solid | 2183K | 3680K | 6 g/L | Transition metal | 1801 |
| 24 | Cr | Chromium | 51.996 u | [Ar]3d5 4s1 | 1.66 | 189 | 0.666 eV | +6, +3, +2 | 6.767 eV | Solid | 2180K | 2944K | 7.15 g/L | Transition metal | 1797 |
| 25 | Mn | Manganese | 54.93804 u | [Ar]4s2 3d5 | 1.55 | 197 | +7, +4, +3, +2 | 7.434 eV | Solid | 1519K | 2334K | 7.3 g/L | Transition metal | 1774 | |
| 26 | Fe | Iron | 55.84 u | [Ar]4s2 3d6 | 1.83 | 194 | 0.163 eV | +3, +2 | 7.902 eV | Solid | 1811K | 3134K | 7.874 g/L | Transition metal | Ancient |
| 27 | Co | Cobalt | 58.93319 u | [Ar]4s2 3d7 | 1.88 | 192 | 0.661 eV | +3, +2 | 7.881 eV | Solid | 1768K | 3200K | 8.86 g/L | Transition metal | 1735 |
| 28 | Ni | Nickel | 58.693 u | [Ar]4s2 3d8 | 1.91 | 163 | 1.156 eV | +3, +2 | 7.64 eV | Solid | 1728K | 3186K | 8.912 g/L | Transition metal | 1751 |
| 29 | Cu | Copper | 63.55 u | [Ar]4s1 3d10 | 1.9 | 140 | 1.228 eV | +2, +1 | 7.726 eV | Solid | 1357.77K | 2835K | 8.933 g/L | Transition metal | Ancient |
| 30 | Zn | Zinc | 65.4 u | [Ar]4s2 3d10 | 1.65 | 139 | 2 | 9.394 eV | Solid | 692.68K | 1180K | 7.134 g/L | Transition metal | 1746 | |
| 31 | Ga | Gallium | 69.723 u | [Ar]4s2 3d10 4p1 | 1.81 | 187 | 0.3 eV | 3 | 5.999 eV | Solid | 302.91K | 2477K | 5.91 g/L | Post-transition metal | 1875 |
| 32 | Ge | Germanium | 72.63 u | [Ar]4s2 3d10 4p2 | 2.01 | 211 | 1.35 eV | +4, +2 | 7.9 eV | Solid | 1211.4K | 3106K | 5.323 g/L | Metalloid | 1886 |
| 33 | As | Arsenic | 74.92159 u | [Ar]4s2 3d10 4p3 | 2.18 | 185 | 0.81 eV | +5, +3, -3 | 9.815 eV | Solid | 1090K | 887K | 5.776 g/L | Metalloid | Ancient |
| 34 | Se | Selenium | 78.97 u | [Ar]4s2 3d10 4p4 | 2.55 | 190 | 2.021 eV | +6, +4, -2 | 9.752 eV | Solid | 493.65K | 958K | 4.809 g/L | Nonmetal | 1817 |
| 35 | Br | Bromine | 79.9 u | [Ar]4s2 3d10 4p5 | 2.96 | 183 | 3.365 eV | +5, +1, -1 | 11.814 eV | Liquid | 265.95K | 331.95K | 3.11 g/L | Halogen | 1826 |
| 36 | Kr | Krypton | 83.8 u | [Ar]4s2 3d10 4p6 | 3 | 202 | 0 | 14 eV | Gas | 115.79K | 119.93K | 0.003733 g/L | Noble gas | 1898 | |
| 37 | Rb | Rubidium | 85.468 u | [Kr]5s1 | 0.82 | 303 | 0.468 eV | 1 | 4.177 eV | Solid | 312.46K | 961K | 1.53 g/L | Alkali metal | 1861 |
| 38 | Sr | Strontium | 87.62 u | [Kr]5s2 | 0.95 | 249 | 2 | 5.695 eV | Solid | 1050K | 1655K | 2.64 g/L | Alkaline earth metal | 1790 | |
| 39 | Y | Yttrium | 88.90584 u | [Kr]5s2 4d1 | 1.22 | 219 | 0.307 eV | 3 | 6.217 eV | Solid | 1795K | 3618K | 4.47 g/L | Transition metal | 1794 |
| 40 | Zr | Zirconium | 91.22 u | [Kr]5s2 4d2 | 1.33 | 186 | 0.426 eV | 4 | 6.634 eV | Solid | 2128K | 4682K | 6.52 g/L | Transition metal | 1789 |
| 41 | Nb | Niobium | 92.90637 u | [Kr]5s1 4d4 | 1.6 | 207 | 0.893 eV | +5, +3 | 6.759 eV | Solid | 2750K | 5017K | 8.57 g/L | Transition metal | 1801 |
| 42 | Mo | Molybdenum | 95.95 u | [Kr]5s1 4d5 | 2.16 | 209 | 0.746 eV | 6 | 7.092 eV | Solid | 2896K | 4912K | 10.2 g/L | Transition metal | 1778 |
| 43 | Tc | Technetium | 96.90636 u | [Kr]5s2 4d5 | 1.9 | 209 | 0.55 eV | +7, +6, +4 | 7.28 eV | Solid | 2430K | 4538K | 11 g/L | Transition metal | 1937 |
| 44 | Ru | Ruthenium | 101.1 u | [Kr]5s1 4d7 | 2.2 | 207 | 1.05 eV | 3 | 7.361 eV | Solid | 2607K | 4423K | 12.1 g/L | Transition metal | 1827 |
| 45 | Rh | Rhodium | 102.9055 u | [Kr]5s1 4d8 | 2.28 | 195 | 1.137 eV | 3 | 7.459 eV | Solid | 2237K | 3968K | 12.4 g/L | Transition metal | 1803 |
| 46 | Pd | Palladium | 106.42 u | [Kr]4d10 | 2.2 | 202 | 0.557 eV | +3, +2 | 8.337 eV | Solid | 1828.05K | 3236K | 12 g/L | Transition metal | 1803 |
| 47 | Ag | Silver | 107.868 u | [Kr]5s1 4d10 | 1.93 | 172 | 1.302 eV | 1 | 7.576 eV | Solid | 1234.93K | 2435K | 10.501 g/L | Transition metal | Ancient |
| 48 | Cd | Cadmium | 112.41 u | [Kr]5s2 4d10 | 1.69 | 158 | 2 | 8.994 eV | Solid | 594.22K | 1040K | 8.69 g/L | Transition metal | 1817 | |
| 49 | In | Indium | 114.818 u | [Kr]5s2 4d10 5p1 | 1.78 | 193 | 0.3 eV | 3 | 5.786 eV | Solid | 429.75K | 2345K | 7.31 g/L | Post-transition metal | 1863 |
| 50 | Sn | Tin | 118.71 u | [Kr]5s2 4d10 5p2 | 1.96 | 217 | 1.2 eV | +4, +2 | 7.344 eV | Solid | 505.08K | 2875K | 7.287 g/L | Post-transition metal | Ancient |
| 51 | Sb | Antimony | 121.76 u | [Kr]5s2 4d10 5p3 | 2.05 | 206 | 1.07 eV | +5, +3, -3 | 8.64 eV | Solid | 903.78K | 1860K | 6.685 g/L | Metalloid | Ancient |
| 52 | Te | Tellurium | 127.6 u | [Kr]5s2 4d10 5p4 | 2.1 | 206 | 1.971 eV | +6, +4, -2 | 9.01 eV | Solid | 722.66K | 1261K | 6.232 g/L | Metalloid | 1782 |
| 53 | I | Iodine | 126.9045 u | [Kr]5s2 4d10 5p5 | 2.66 | 198 | 3.059 eV | +7, +5, +1, -1 | 10.451 eV | Solid | 386.85K | 457.55K | 4.93 g/L | Halogen | 1811 |
| 54 | Xe | Xenon | 131.29 u | [Kr]5s2 4d10 5p6 | 2.6 | 216 | 0 | 12.13 eV | Gas | 161.36K | 165.03K | 0.005887 g/L | Noble gas | 1898 | |
| 55 | Cs | Cesium | 132.905452 u | [Xe]6s1 | 0.79 | 343 | 0.472 eV | 1 | 3.894 eV | Solid | 301.59K | 944K | 1.93 g/L | Alkali metal | 1860 |
| 56 | Ba | Barium | 137.33 u | [Xe]6s2 | 0.89 | 268 | 2 | 5.212 eV | Solid | 1000K | 2170K | 3.62 g/L | Alkaline earth metal | 1808 | |
| 57 | La | Lanthanum | 138.9055 u | [Xe]6s2 5d1 | 1.1 | 240 | 0.5 eV | 3 | 5.577 eV | Solid | 1191K | 3737K | 6.15 g/L | Lanthanide | 1839 |
| 58 | Ce | Cerium | 140.116 u | [Xe]6s2 4f1 5d1 | 1.12 | 235 | 0.5 eV | +4, +3 | 5.539 eV | Solid | 1071K | 3697K | 6.77 g/L | Lanthanide | 1803 |
| 59 | Pr | Praseodymium | 140.90766 u | [Xe]6s2 4f3 | 1.13 | 239 | 3 | 5.464 eV | Solid | 1204K | 3793K | 6.77 g/L | Lanthanide | 1885 | |
| 60 | Nd | Neodymium | 144.24 u | [Xe]6s2 4f4 | 1.14 | 229 | 3 | 5.525 eV | Solid | 1294K | 3347K | 7.01 g/L | Lanthanide | 1885 | |
| 61 | Pm | Promethium | 144.91276 u | [Xe]6s2 4f5 | 236 | 3 | 5.55 eV | Solid | 1315K | 3273K | 7.26 g/L | Lanthanide | 1945 | ||
| 62 | Sm | Samarium | 150.4 u | [Xe]6s2 4f6 | 1.17 | 229 | +3, +2 | 5.644 eV | Solid | 1347K | 2067K | 7.52 g/L | Lanthanide | 1879 | |
| 63 | Eu | Europium | 151.964 u | [Xe]6s2 4f7 | 233 | +3, +2 | 5.67 eV | Solid | 1095K | 1802K | 5.24 g/L | Lanthanide | 1901 | ||
| 64 | Gd | Gadolinium | 157.2 u | [Xe]6s2 4f7 5d1 | 1.2 | 237 | 3 | 6.15 eV | Solid | 1586K | 3546K | 7.9 g/L | Lanthanide | 1880 | |
| 65 | Tb | Terbium | 158.92535 u | [Xe]6s2 4f9 | 221 | 3 | 5.864 eV | Solid | 1629K | 3503K | 8.23 g/L | Lanthanide | 1843 | ||
| 66 | Dy | Dysprosium | 162.5 u | [Xe]6s2 4f10 | 1.22 | 229 | 3 | 5.939 eV | Solid | 1685K | 2840K | 8.55 g/L | Lanthanide | 1886 | |
| 67 | Ho | Holmium | 164.93033 u | [Xe]6s2 4f11 | 1.23 | 216 | 3 | 6.022 eV | Solid | 1747K | 2973K | 8.8 g/L | Lanthanide | 1878 | |
| 68 | Er | Erbium | 167.26 u | [Xe]6s2 4f12 | 1.24 | 235 | 3 | 6.108 eV | Solid | 1802K | 3141K | 9.07 g/L | Lanthanide | 1843 | |
| 69 | Tm | Thulium | 168.93422 u | [Xe]6s2 4f13 | 1.25 | 227 | 3 | 6.184 eV | Solid | 1818K | 2223K | 9.32 g/L | Lanthanide | 1879 | |
| 70 | Yb | Ytterbium | 173.05 u | [Xe]6s2 4f14 | 242 | +3, +2 | 6.254 eV | Solid | 1092K | 1469K | 6.9 g/L | Lanthanide | 1878 | ||
| 71 | Lu | Lutetium | 174.9668 u | [Xe]6s2 4f14 5d1 | 1.27 | 221 | 3 | 5.426 eV | Solid | 1936K | 3675K | 9.84 g/L | Lanthanide | 1907 | |
| 72 | Hf | Hafnium | 178.49 u | [Xe]6s2 4f14 5d2 | 1.3 | 212 | 4 | 6.825 eV | Solid | 2506K | 4876K | 13.3 g/L | Transition metal | 1923 | |
| 73 | Ta | Tantalum | 180.9479 u | [Xe]6s2 4f14 5d3 | 1.5 | 217 | 0.322 eV | 5 | 7.89 eV | Solid | 3290K | 5731K | 16.4 g/L | Transition metal | 1802 |
| 74 | W | Tungsten | 183.84 u | [Xe]6s2 4f14 5d4 | 2.36 | 210 | 0.815 eV | 6 | 7.98 eV | Solid | 3695K | 5828K | 19.3 g/L | Transition metal | 1783 |
| 75 | Re | Rhenium | 186.207 u | [Xe]6s2 4f14 5d5 | 1.9 | 217 | 0.15 eV | +7, +6, +4 | 7.88 eV | Solid | 3459K | 5869K | 20.8 g/L | Transition metal | 1925 |
| 76 | Os | Osmium | 190.2 u | [Xe]6s2 4f14 5d6 | 2.2 | 216 | 1.1 eV | +4, +3 | 8.7 eV | Solid | 3306K | 5285K | 22.57 g/L | Transition metal | 1803 |
| 77 | Ir | Iridium | 192.22 u | [Xe]6s2 4f14 5d7 | 2.2 | 202 | 1.565 eV | +4, +3 | 9.1 eV | Solid | 2719K | 4701K | 22.42 g/L | Transition metal | 1803 |
| 78 | Pt | Platinum | 195.08 u | [Xe]6s1 4f14 5d9 | 2.28 | 209 | 2.128 eV | +4, +2 | 9 eV | Solid | 2041.55K | 4098K | 21.46 g/L | Transition metal | 1735 |
| 79 | Au | Gold | 196.96657 u | [Xe]6s1 4f14 5d10 | 2.54 | 166 | 2.309 eV | +3, +1 | 9.226 eV | Solid | 1337.33K | 3129K | 19.282 g/L | Transition metal | Ancient |
| 80 | Hg | Mercury | 200.59 u | [Xe]6s2 4f14 5d10 | 2 | 209 | +2, +1 | 10.438 eV | Liquid | 234.32K | 629.88K | 13.5336 g/L | Transition metal | Ancient | |
| 81 | Tl | Thallium | 204.383 u | [Xe]6s2 4f14 5d10 6p1 | 1.62 | 196 | 0.2 eV | +3, +1 | 6.108 eV | Solid | 577K | 1746K | 11.8 g/L | Post-transition metal | 1861 |
| 82 | Pb | Lead | 207 u | [Xe]6s2 4f14 5d10 6p2 | 2.33 | 202 | 0.36 eV | +4, +2 | 7.417 eV | Solid | 600.61K | 2022K | 11.342 g/L | Post-transition metal | Ancient |
| 83 | Bi | Bismuth | 208.9804 u | [Xe]6s2 4f14 5d10 6p3 | 2.02 | 207 | 0.946 eV | +5, +3 | 7.289 eV | Solid | 544.55K | 1837K | 9.807 g/L | Post-transition metal | 1753 |
| 84 | Po | Polonium | 208.98243 u | [Xe]6s2 4f14 5d10 6p4 | 2 | 197 | 1.9 eV | +4, +2 | 8.417 eV | Solid | 527K | 1235K | 9.32 g/L | Metalloid | 1898 |
| 85 | At | Astatine | 209.98715 u | [Xe]6s2 4f14 5d10 6p5 | 2.2 | 202 | 2.8 eV | 7, 5, 3, 1, -1 | 9.5 eV | Solid | 575K | 7 g/L | Halogen | 1940 | |
| 86 | Rn | Radon | 222.01758 u | [Xe]6s2 4f14 5d10 6p6 | 220 | 0 | 10.745 eV | Gas | 202K | 211.45K | 0.00973 g/L | Noble gas | 1900 | ||
| 87 | Fr | Francium | 223.01973 u | [Rn]7s1 | 0.7 | 348 | 0.47 eV | 1 | 3.9 eV | Solid | 300K | Alkali metal | 1939 | ||
| 88 | Ra | Radium | 226.02541 u | [Rn]7s2 | 0.9 | 283 | 2 | 5.279 eV | Solid | 973K | 1413K | 5 g/L | Alkaline earth metal | 1898 | |
| 89 | Ac | Actinium | 227.02775 u | [Rn]7s2 6d1 | 1.1 | 260 | 3 | 5.17 eV | Solid | 1324K | 3471K | 10.07 g/L | Actinide | 1899 | |
| 90 | Th | Thorium | 232.038 u | [Rn]7s2 6d2 | 1.3 | 237 | 4 | 6.08 eV | Solid | 2023K | 5061K | 11.72 g/L | Actinide | 1828 | |
| 91 | Pa | Protactinium | 231.03588 u | [Rn]7s2 5f2 6d1 | 1.5 | 243 | +5, +4 | 5.89 eV | Solid | 1845K | 15.37 g/L | Actinide | 1913 | ||
| 92 | U | Uranium | 238.0289 u | [Rn]7s2 5f3 6d1 | 1.38 | 240 | +6, +5, +4, +3 | 6.194 eV | Solid | 1408K | 4404K | 18.95 g/L | Actinide | 1789 | |
| 93 | Np | Neptunium | 237.048172 u | [Rn]7s2 5f4 6d1 | 1.36 | 221 | +6, +5, +4, +3 | 6.266 eV | Solid | 917K | 4175K | 20.25 g/L | Actinide | 1940 | |
| 94 | Pu | Plutonium | 244.0642 u | [Rn]7s2 5f6 | 1.28 | 243 | +6, +5, +4, +3 | 6.06 eV | Solid | 913K | 3501K | 19.84 g/L | Actinide | 1940 | |
| 95 | Am | Americium | 243.06138 u | [Rn]7s2 5f7 | 1.3 | 244 | +6, +5, +4, +3 | 5.993 eV | Solid | 1449K | 2284K | 13.69 g/L | Actinide | 1944 | |
| 96 | Cm | Curium | 247.07035 u | [Rn]7s2 5f7 6d1 | 1.3 | 245 | 3 | 6.02 eV | Solid | 1618K | 3400K | 13.51 g/L | Actinide | 1944 | |
| 97 | Bk | Berkelium | 247.07031 u | [Rn]7s2 5f9 | 1.3 | 244 | +4, +3 | 6.23 eV | Solid | 1323K | 14 g/L | Actinide | 1949 | ||
| 98 | Cf | Californium | 251.07959 u | [Rn]7s2 5f10 | 1.3 | 245 | 3 | 6.3 eV | Solid | 1173K | Actinide | 1950 | |||
| 99 | Es | Einsteinium | 252.083 u | [Rn]7s2 5f11 | 1.3 | 245 | 3 | 6.42 eV | Solid | 1133K | Actinide | 1952 | |||
| 100 | Fm | Fermium | 257.09511 u | [Rn] 5f12 7s2 | 1.3 | 3 | 6.5 eV | Solid | 1800K | Actinide | 1952 | ||||
| 101 | Md | Mendelevium | 258.09843 u | [Rn]7s2 5f13 | 1.3 | +3, +2 | 6.58 eV | Solid | 1100K | Actinide | 1955 | ||||
| 102 | No | Nobelium | 259.101 u | [Rn]7s2 5f14 | 1.3 | +3, +2 | 6.65 eV | Solid | 1100K | Actinide | 1957 | ||||
| 103 | Lr | Lawrencium | 266.12 u | [Rn]7s2 5f14 6d1 | 1.3 | 3 | Solid | 1900K | Actinide | 1961 | |||||
| 104 | Rf | Rutherfordium | 267.122 u | [Rn]7s2 5f14 6d2 | 4 | Solid | Transition metal | 1964 | |||||||
| 105 | Db | Dubnium | 268.126 u | [Rn]7s2 5f14 6d3 | 5, 4, 3 | Solid | Transition metal | 1967 | |||||||
| 106 | Sg | Seaborgium | 269.128 u | [Rn]7s2 5f14 6d4 | 6, 5, 4, 3, 0 | Solid | Transition metal | 1974 | |||||||
| 107 | Bh | Bohrium | 270.133 u | [Rn]7s2 5f14 6d5 | 7, 5, 4, 3 | Solid | Transition metal | 1976 | |||||||
| 108 | Hs | Hassium | 269.1336 u | [Rn]7s2 5f14 6d6 | 8, 6, 5, 4, 3, 2 | Solid | Transition metal | 1984 | |||||||
| 109 | Mt | Meitnerium | 277.154 u | [Rn]7s2 5f14 6d7 (calculated) | 9, 8, 6, 4, 3, 1 | Solid | Transition metal | 1982 | |||||||
| 110 | Ds | Darmstadtium | 282.166 u | [Rn]7s2 5f14 6d8 (predicted) | 8, 6, 4, 2, 0 | Expected to be a Solid | Transition metal | 1994 | |||||||
| 111 | Rg | Roentgenium | 282.169 u | [Rn]7s2 5f14 6d9 (predicted) | 5, 3, 1, -1 | Expected to be a Solid | Transition metal | 1994 | |||||||
| 112 | Cn | Copernicium | 286.179 u | [Rn]7s2 5f14 6d10 (predicted) | 2, 1, 0 | Expected to be a Solid | Transition metal | 1996 | |||||||
| 113 | Nh | Nihonium | 286.182 u | [Rn]5f14 6d10 7s2 7p1 (predicted) | Expected to be a Solid | Post-transition metal | 2004 | ||||||||
| 114 | Fl | Flerovium | 290.192 u | [Rn]7s2 7p2 5f14 6d10 (predicted) | 6, 4,2, 1, 0 | Expected to be a Solid | Post-transition metal | 1998 | |||||||
| 115 | Mc | Moscovium | 290.196 u | [Rn]7s2 7p3 5f14 6d10 (predicted) | 3, 1 | Expected to be a Solid | Post-transition metal | 2003 | |||||||
| 116 | Lv | Livermorium | 293.205 u | [Rn]7s2 7p4 5f14 6d10 (predicted) | +4, +2, -2 | Expected to be a Solid | Post-transition metal | 2000 | |||||||
| 117 | Ts | Tennessine | 294.211 u | [Rn]7s2 7p5 5f14 6d10 (predicted) | +5, +3, +1, -1 | Expected to be a Solid | Halogen | 2010 | |||||||
| 118 | Og | Oganesson | 295.216 u | [Rn]7s2 7p6 5f14 6d10 (predicted) | +6, +4, +2, +1, 0, -1 | Expected to be a Gas | Noble gas | 2006 |
You can find the comprehensive details of Modern Periodic law here.
READ MORE



